Control how you accelerate your clinical trials
Take full command of every aspect of your clinical trials and research with Zelta.
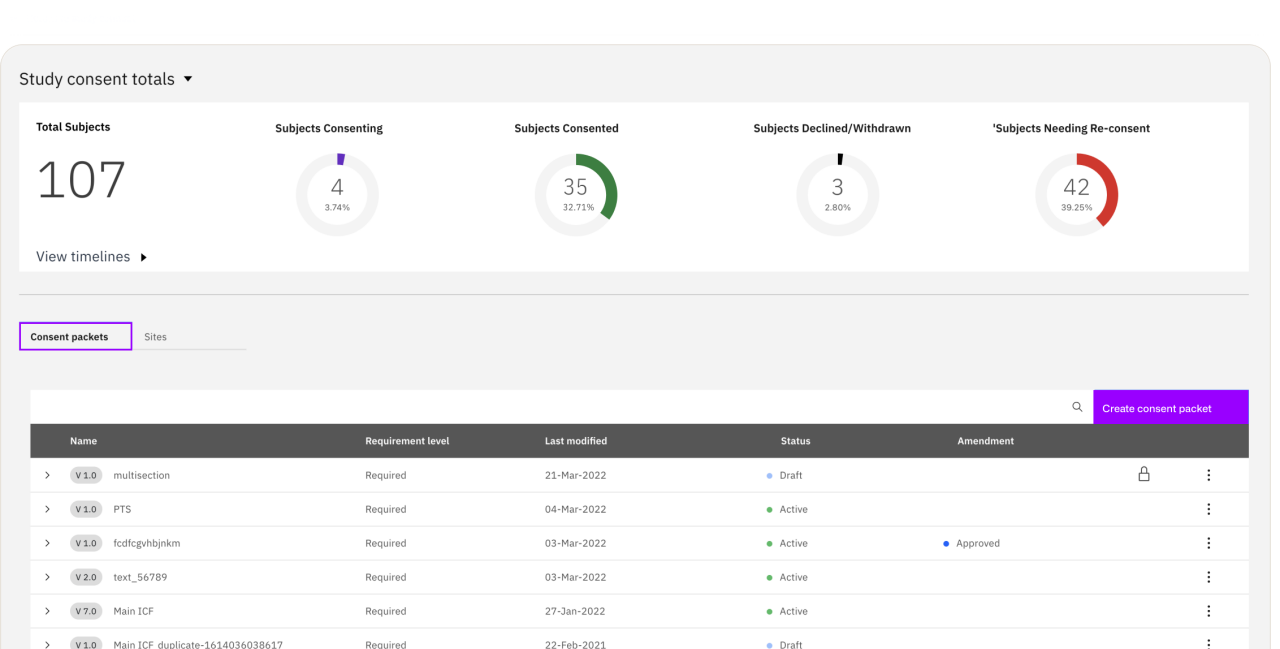
Zelta, a unified clinical data management and acquisition platform with customizable modules, can be tailored to the meet the unique needs of your clinical trials to accelerate outcomes.